Lucozade Ribena Suntory (LRS) is reducing the sugars and carbohydrate levels of their drinks by approximately 50%. From April 2017, new products will start appearing on shelves and for a time new and old formulas may be present at the same time. It is essential that consumers check the label. Lucozade Energy original and orange will appear from April followed by other products later in the year.
These changes may impact on Health Care Professionals who:
Give dietary advice to people with diabetes who currently drink Lucozade Energy, Ribena or Orangina to manage their blood sugar levels and may use Lucozade Energy Original as the source of glucose for undertaking glucose tolerance tests;
Work with people with Phenylketonuria (PKU), who will need to check the ingredients label as some products will contain the sweetener aspartame, which is a source of phenylalanine. These products will carry a warning on the back label as a legal requirement;
Give dietary advice to people with other metabolic conditions who may use Lucozade Energy, Ribena or Orangina
Health Care Professionals and their patients will need to check the nutritional information label located on the back of the pack. As an example please see new and old product label for Lucozade Energy original below.
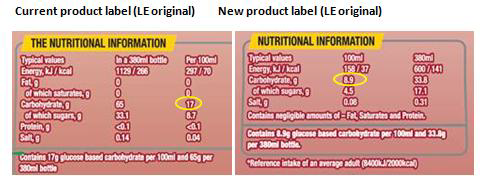
Lucozade Ribena Suntory has prepared a selection of communication materials to advise consumers and health care professionals of the product formulation changes. Their consumer care team can be contacted at hcp.enquiry@lrsuntory.com.
A poster communicating the formulation changes is available to download from www.lrsuntory.com/health.