The most significant effect of the new regulations (due to come into effect on May 5th) is in relation to benzodiazepines and z-drugs.
Benzodiazepines will be removed from the Misuse of Drugs Exemption Order and, as a result, the restrictions in place on the possession of controlled drugs will apply to all benzodiazepines.
The benzodiazepines previously found in Schedule 4 of the 1988 Regulations are now found in a new Schedule 4 Part 1 of the new 2017 Regulations. The "z-drugs" zopiclone, zolpidem and zaleplon will also be listed in Schedule 4.
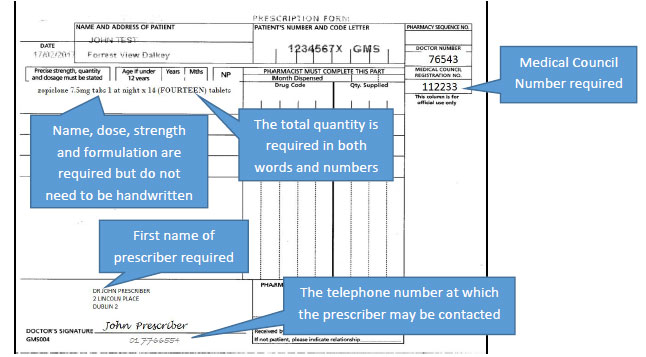
The specific criteria to be included on a prescription for Schedule 2 and 3 controlled drugs will now also apply to controlled drugs in Schedule 4 Part 1 i.e. most benzodiazepines and z-drugs:
However, for controlled drugs in Schedule 4 Part 1 only, these will not be required to be handwritten. The requirements for these specific criteria to be specified in the prescriber's handwriting will also not apply to prescriptions for Methadone.
Prescriptions for controlled drugs in Schedule 4 Part 1 can be repeated if the prescriber clearly marks the prescription accordingly.
Examples of prescriptions are as follows.
A prescription for Schedule 2 and 3 controlled drugs would look as follows, with the changes and key points highlighted.
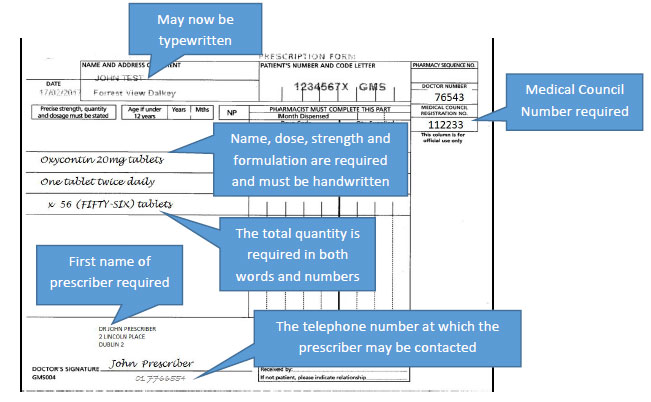
The requirements above also apply for controlled drugs in Schedule 4 Part 1, however the prescription in this case will not be required to be handwritten.