New Misuse of Drugs Regulations 2017 – Advice for GP prescribing
The most significant effect of the new regulations (due to come into effect on May 5th) is in relation to benzodiazepines and z-drugs.
Benzodiazepines will be removed from the Misuse of Drugs Exemption Order and, as a result, the restrictions in place on the possession of controlled drugs will apply to all benzodiazepines.
The benzodiazepines previously found in Schedule 4 of the 1988 Regulations are now found in a new Schedule 4 Part 1 of the new 2017 Regulations. The "z-drugs" zopiclone, zolpidem and zaleplon will also be listed in Schedule 4.
Changes to the 'Form of Prescriptions' and 'Supply on Prescription'
(previously Regulations 13 and 14 of the 1988 Regulations)
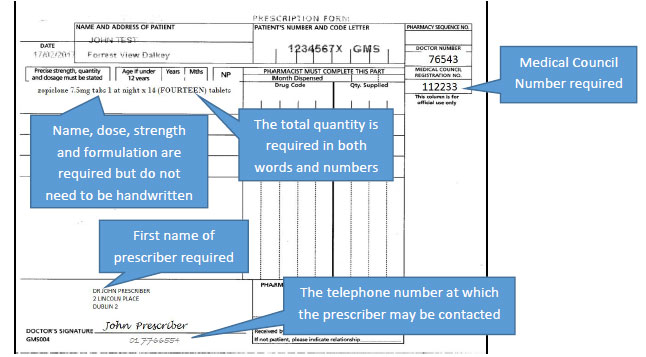
- Inclusion of the first name of the prescriber on the prescription
- Inclusion of the prescriber's registration number on the prescription
- The name of the controlled drug to be prescribed must be included on the prescription i.e. either the common/generic name or the proprietary/brand name of the preparation
- The total quantity of the controlled drug to be prescribed must be written in words and figures
- The name and address of the person for whom the treatment is issued shall no longer be required to be handwritten for a controlled drug in Schedule 2 or 3. NB an addressograph (adhesive label) will not fulfil the requirement for this information to be indelible.
- The handwriting requirements for the specific criteria to be included on a prescription for Schedule 2 or 3 controlled drugs will continue to apply.
Additional prescribing requirements for Schedule 4 Part 1 controlled drugs
The specific criteria to be included on a prescription for Schedule 2 and 3 controlled drugs will now also apply to controlled drugs in Schedule 4 Part 1 i.e. most benzodiazepines and z-drugs:
- The name of the drug
- Dose
- Pharmaceutical form
- Strength (where appropriate)
- The total quantity written in both words and figures (numbers) (e.g. 2/52 or 1/12 will not suffice)
However, for controlled drugs in Schedule 4 Part 1 only, these will not be required to be handwritten. The requirements for these specific criteria to be specified in the prescriber's handwriting will also not apply to prescriptions for Methadone.
Prescriptions for controlled drugs in Schedule 4 Part 1 can be repeated if the prescriber clearly marks the prescription accordingly.
Examples of prescriptions are as follows.
A prescription for Schedule 2 and 3 controlled drugs would look as follows, with the changes and key points highlighted.
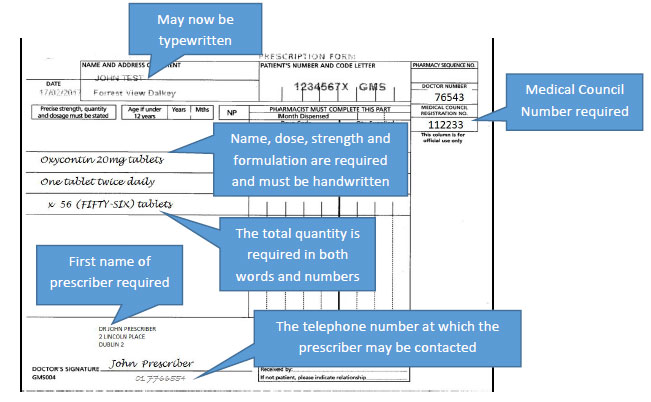
The requirements above also apply for controlled drugs in Schedule 4 Part 1, however the prescription in this case will not be required to be handwritten.